In the complex world of medical device development, it’s easy to get lost in the noise of different stakeholders’ demands and expectations.
It’s common for everyone to have strong opinions about what they believe is essential, but the key lies in our ability to discern true needs amidst this tumult. In other words, it’s crucial to understand the context of use, the real user needs, and the parameters that should be considered for informed decision-making.
Use Discernment to Understand the Real Need
When developing medical devices, the context of use is the guiding thread throughout the process. A customer may insist that their device be the quietest on the market, but without understanding where and how it will be used, this requirement might become an inconsequential whisper.
Don't Lose Sight of Real User Needs
NEW EBOOK
Demystify the design process with 7 frequently asked questions about UX
From dynamic sketches to immersive role-playing, every discipline have to be involved in this UX approach focused on creating unforgettable interactions 🚀
Discover our insights and elevate your UX design process by downloading our free ebook!
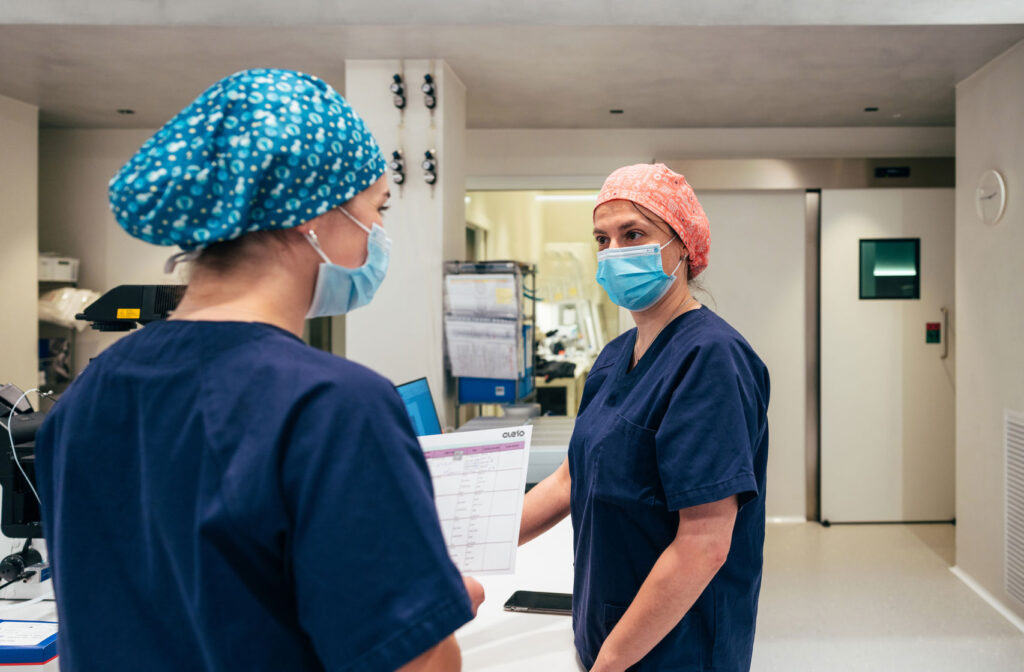
Other Parameters to Consider
Every medical device designer knows that every detail matters. Parameters such as durability, ease of use, and safety are just as important, if not more so, than more obvious features.
Striking a Balance Between Stakeholders' Wishes and Real-World Imperatives
Medical device development is a careful dance between stakeholder wishes and real-world necessities. Understanding the context of use, deciphering real user needs and considering a wide range of parameters are the keys to informed decision-making.

