The multitude of technological devices in our lives has made users increasingly demanding and critical of specialized devices, and for the better. This new reality necessitates placing users at the center of the development process for a new product. Knowing how to listen to, observe, and decode their needs are skills essential to the success of a project and must be considered when building a high-performing design team.
François Gélinas, Director of Design at CLEIO, shares his insights on the role and value of design in medical device development.
Understanding the Role of Design in the Medical Device Development Process
What is User-Centered Design?
User-centered design prioritizes the needs and expectations of end-users from the beginning of the design process. It advocates for thorough research to understand their behaviors and motivations, followed by usability testing to refine the product according to their feedback.
This approach seeks to develop intuitive, accessible, and effective solutions, enhancing the overall user experience.
User Needs: The Core of Design
Design Goes Beyond Aesthetics
All this must be verified as early as possible in the development process to minimize the risk of late-stages, costly changes. Do you see the value now?
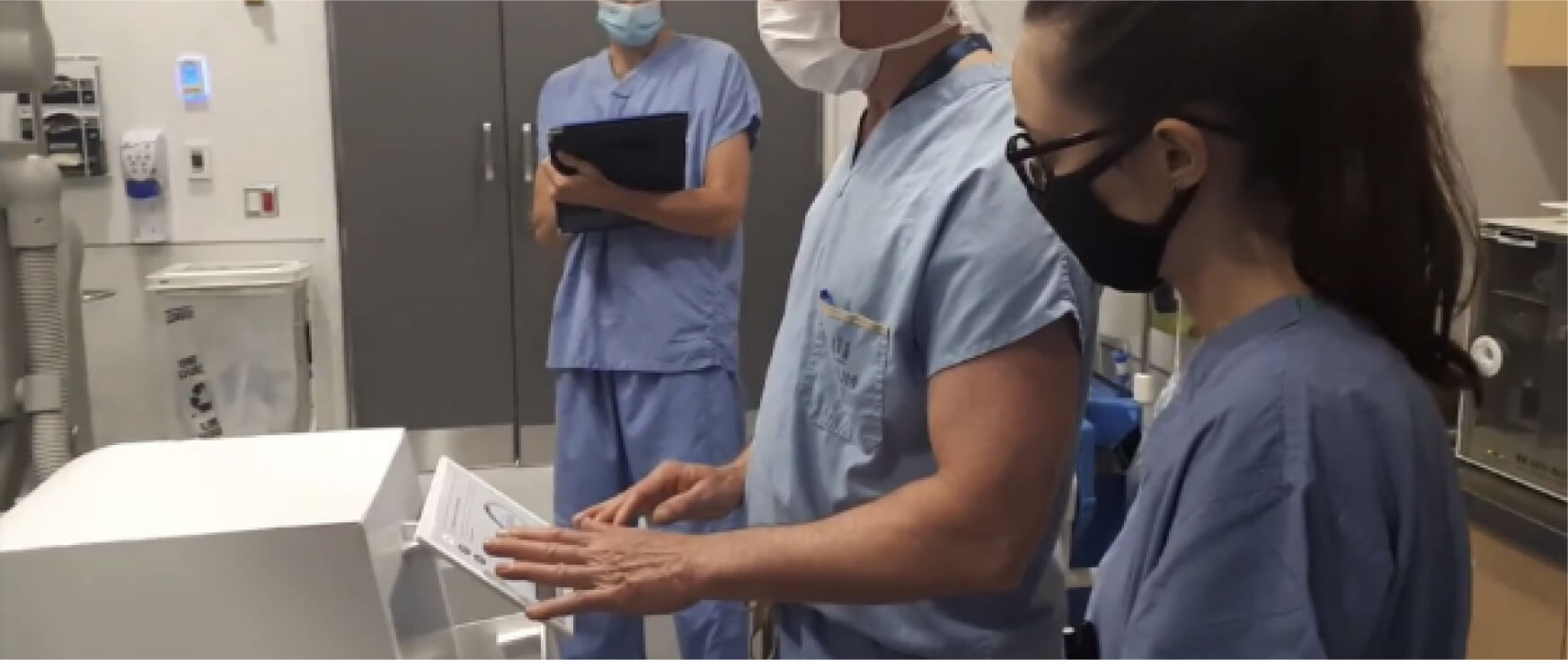
Evaluating Design Value One Test at a Time
Formative evaluations conducted during the development phase can rapidly identify potential usability issues, including risks. They also enhance the design process by emphasizing qualitative, intangible aspects in addition to quantifiable aspects.
Reducing Risks
Often, this involves mobilizing multiple experts within the identified user population, such as doctors and specialists, to conduct formative evaluations under conditions that closely mimic real-life use. Consequently, the investment in time and human resources is significant, necessitating meticulous planning beforehand.
In this context, the value of design is reflected in satisfactory results, appreciated not just by the developers, but also by all involved parties. For instance, an emergency doctor asked to test a new interventional device will be especially engaged if they find the product to be well-conceived and efficient from the outset.
François Gélinas
Director of Design at CLEIO
Enhancing the Medical Device Design Process
Beyond this valuable data, it’s also crucial to understand how to interpret general appreciation, which isn’t easily quantified on a scale of 1 to 10. This involves recognizing non-verbal cues: a lingering look on a detail, a spontaneous comment of approval, or, conversely, a questioning eyebrow.
Collaboration Between Design and Engineering Teams
For the concept development process to succeed, constituting the right multidisciplinary team is crucial. This approach ensures comprehensive coverage of all aspects and minimizes technical risks. Hence, we hold the belief that Design is everyone’s responsibility.
Embracing a Design Mindset Right from the Definition Phase
In the Definition phase of our IDEAL development process, adopting a Design mindset is crucial. Whether you are a quality assurance expert, an electronics, mechanical, or software engineer, embracing a design mindset in the context of solution-finding offers an undeniable advantage.
We’ve come to understand that ‘Design’ is not exclusively the domain of industrial or UX/UI designers, whose expertise is derived from their training. It’s inconceivable for such complex products to be conceived by these experts alone. When individuals from other disciplines also embrace the designer’s mindset, it fosters the development of robust and compelling concepts.
The Design Defines the ‘Why’
The Engineering Defines the ‘How’

Aligning Design with Business Objectives
In light of this new reality, the value proposition of the product must align with the company’s objectives. Design also plays a crucial role in understanding these concerns. It can be risky to adhere strictly to the doctrine of ‘putting the user first’ at the expense of commercial realities and budgetary constraints.
It’s all about finding the right balance. Design, through Innovation Strategy activities, should empower the company to make well-informed decisions by considering all essential data necessary to deliver maximum impact in the market.
Design is Everyone's Responsibility
At CLEIO, user-centered design isn’t just for industrial and UX/UI designers. It also extends to all disciplines involved in design, development, quality control, and testing.