Rewatch the Webinar
Do it by Design: How Usability Brings Value to Risk Controls
- Session Recording: 50 min
Are you still questioning if usability can truly impact medical device design? The answer is yes. Specifically, it plays a crucial role in controlling risks throughout the development process.
Guided by the IEC 62366-1 standard, usability engineering primarily focuses on the risks of use error, in order to design safer, user-centered products.
Asra Saleh is a risk management expert in CLEIO’s Quality Assurance team. A few months ago, she hosted a webinar for Greenlight Guru discussing the role and impact of usability in risk control.
Enhancing Medical Device Safe Use Through Usability Engineering
Use errors are the primary cause of incidents related to medical devices. To avoid them, it is necessary to pay more attention to human factors and usability. To gain a clearer understanding of the role usability can play in medical device design, let’s take an example of a scenario that could occur in real life.
How could this error have been avoided? By focusing on users and the potential use-related risks when designing the infusion pump. For instance, setting dose limits could have been considered. This is where usability engineering plays a crucial role.
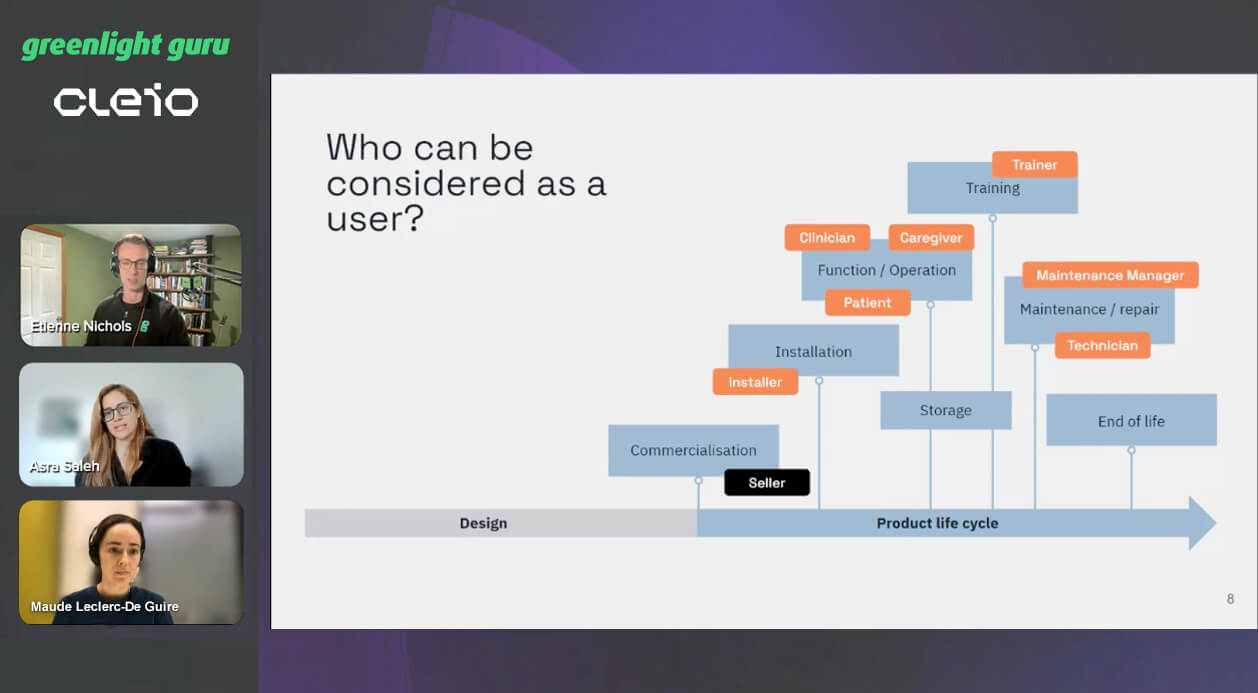
Medical Device Use-Related Risks
The goal of usability engineering is to identify the potential use-related risks in order to mitigate them and make them acceptable: if zero risk doesn’t exist, our aim is to make the use as safe as possible.
User-Centered Design Cycle
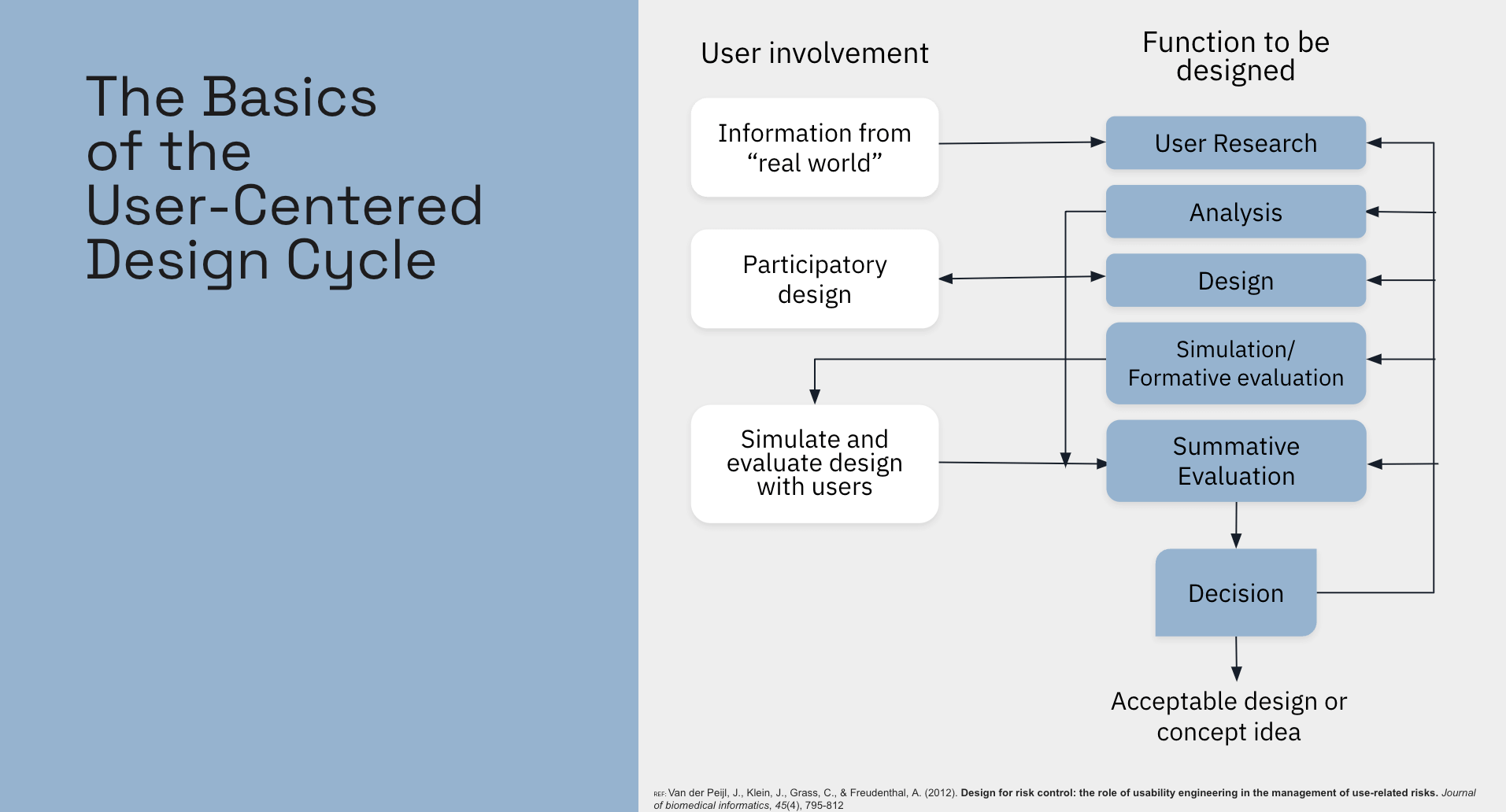
How to Design for Risk Control?
Through the example of the infusion pump, we’ve seen how a poorly designed user interface can lead to use-related risks that can negatively impact patient safety. This underscores the need to integrate usability engineering early in the development process, starting from the concept phase of the medical device. Incorporating it too late in the process would significantly reduce its effectiveness in controlling risks.
Creating a Checklist for Use-Related Risks
- Technical failures
- Device operating issues
- User skills
- Use environment
- Patient-related scenarios
- Availability of trained personnel
IEC 62366-1 also suggests examining public databases like TPLC (Total Product Life Cycle) to collect information on any recalls that have occurred due to adverse events with similar products.
Applying a Risk Control Approach
- Inherent safety by design and manufacture
- Incorporating protective measures into the medical device itself or its manufacturing process
- Providing safety information (such as written warnings, contraindications, training)
Integrating Usability and Risk Management Activities
ISO 14971 outlines a risk management framework, crucial for medical device development, providing manufacturers with a systematic approach to identify, analyze, evaluate, and control risks associated with the intended use of their products.
Therefore, ISO 14971 is a decision-making process, vital for medical device development problem identification, while IEC 62366-1 is a design and development process, making them complementary.
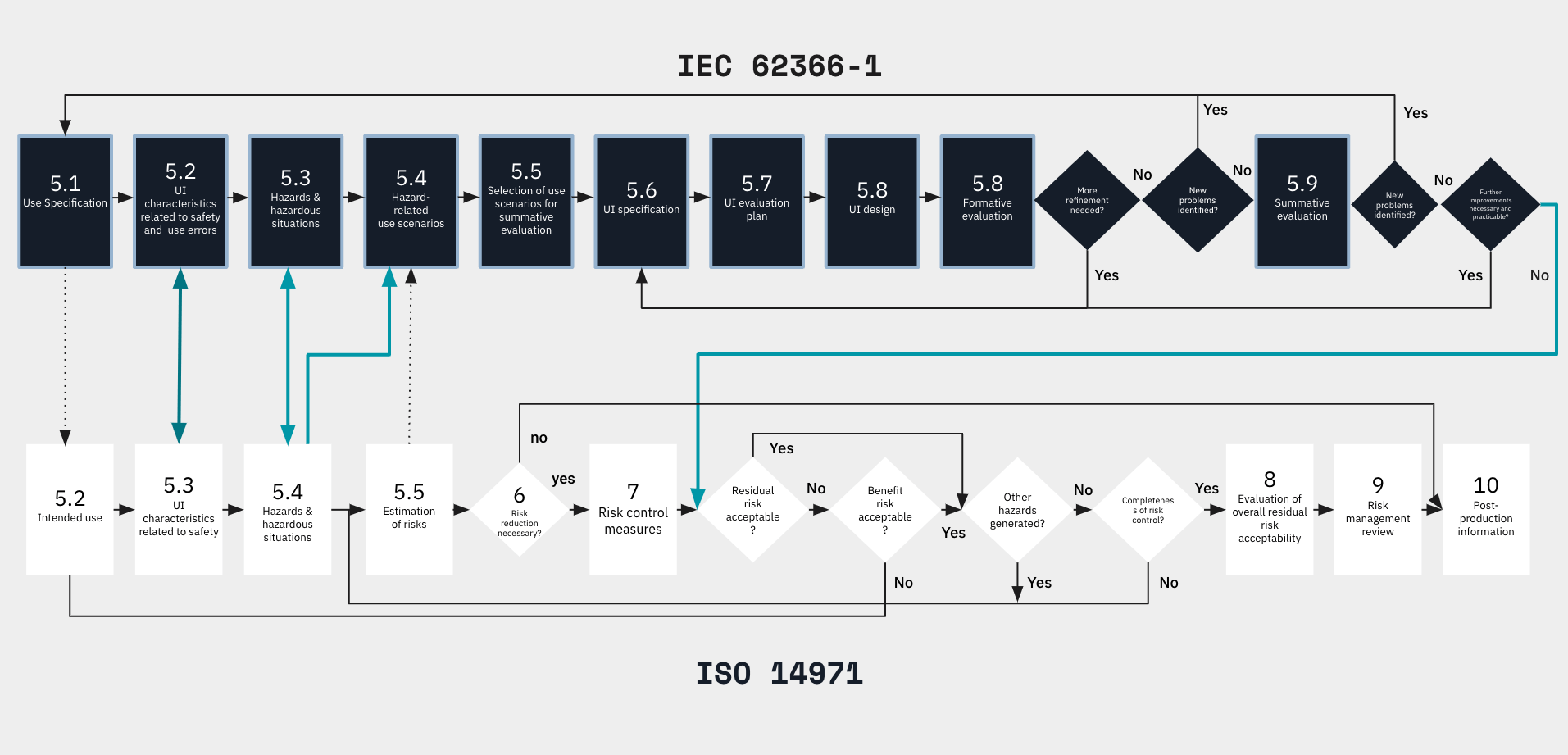
The blue lines in the flowchart illustrate the connection between the usability engineering in medical device design process and the risk management process.
- During the "Analysis" phase (5.2 →5.4), characteristics related to safety, foreseeable hazards, and hazardous situations are identified.
- After the "Evaluation" phase, it is essential to verify if any additional hazards have emerged and if all hazards have been considered.
Applying UX Methods
UX methods are an impressive toolkit available to usability engineers. Each engineer can select the appropriate tool based on the nature of the device being designed.
- User interviews
- Contextual inquiry
- Heuristic evaluations (or ergonomic audits)
- Cognitive walkthrough
- Usability testing
- Understand the context and environment of use
- Define user profiles and understand their needs
- Facilitate brainstorming sessions with other team members who have specific expertise
- Evaluate product usability for informed design decisions
- Improve user experience
When is it Good Enough?
This is the approach we take at CLEIO: our expert Human Factors Engineering team and Quality Assurance team work in close coordination. For the medical devices we develop, this means ensuring that risks associated with the user interface are mitigated and that overall residual risks are acceptable. All of this is done with one goal in mind: to create a product that is safe for both the patient and healthcare professional.